IMAs main roles are:
- to issue marketing authorizations for medicines in Iceland in collaboration with regulatory authorities in the European Economic Area (EEA)
- monitor adverse reactions from medical products
- ensure control and surveillance of the pharmaceutical industry in Iceland
- authorize clinical trials
- decide which medicines are eligible for reimbursement
- monitor medical devices available in Iceland and supervise adverse incidents involving medical devices and
- to contribute to making professional and unbiased information on medicines available to healthcare professionals and consumers.
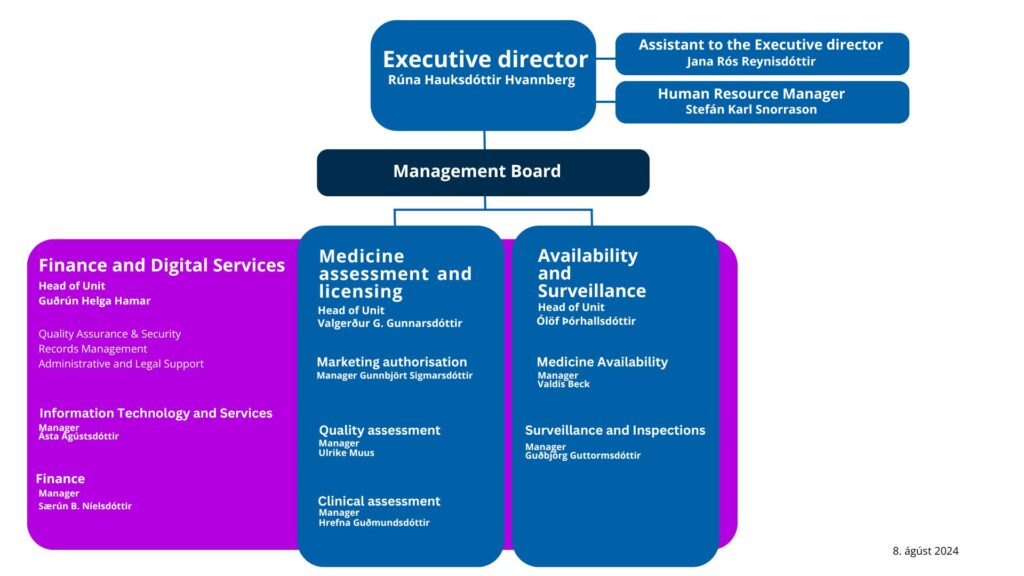
Organization
At IMA there are two core units and one supporting unit.
Core units:
- Medicine assessment and licensing
- Availability and Surveillance
Supporting Unit:
- Finance and Digital Services
Why do medicines need to be assessed by the authorities before they are marketed?
Safety and health issues are the main reasons why medicines are assessed by the authorities before they are placed on the market.
Before a new medicinal product can be placed on the market, the applicant must perform extensive toxicological, quality and clinical studies. The regulatory authorities consequently assess the results of these studies in order to confirm the quality and safety of the medicine. Only then can the medicine be released on the market to the consumers.
Further requirements are made to veterinary medicines on maximum residue limits in animals intended for human consumption.
Close collaboration among medicines authorities in Europe
In order to ensure health protection and free movements of medicines across the EEA, the European Union (EU) has harmonised requirements for research and testing among the Member States. The European Medicines Agency (EMA) located in Amsterdam plays a central role in the network of close collaboration among the medicines authorities, concerning marketing authorisations and surveillance of medicines.
EMA is responsible for the centrally authorised medicines but the individual Member States perform the assessments of applications for national and mutual recognition marketing authorisations.
IMA is a WHO Listed Authority.